
Careers
How to Choose and Use High Purity Chemicals for Your Experiments
In the realm of scientific research, the selection and application of high purity chemicals are paramount to achieving accurate and reproducible results. According to a report by the global market research firm Research and Markets, the high purity chemicals market is projected to grow significantly, reaching $80 billion by 2025, driven by advancements in pharmaceutical, biotechnology, and materials science sectors. This underscores the increasing demand for substances that meet stringent quality standards, allowing scientists and researchers to push the boundaries of innovation.
Dr. Emily Chen, a leading expert in analytical chemistry, emphasizes the importance of high purity chemicals in her field: "The reliability of experimental outcomes relies heavily on the quality of the chemicals used. Impurities can skew results and lead to erroneous conclusions." This statement highlights the critical role that high purity chemicals play in experimental design and execution. Properly selecting these materials is essential for anyone involved in scientific inquiry, whether in academic research or industrial applications, where precision and accuracy are non-negotiable.
In this guide, we will explore how to choose the appropriate high purity chemicals for your specific experiments while addressing considerations such as supplier credibility, quality assurance protocols, and critical handling practices. By adhering to these guidelines, researchers can ensure that their experiments yield the most reliable and valid results, ultimately contributing to the advancement of science and technology.

Understanding High Purity Chemicals and Their Importance in Experiments
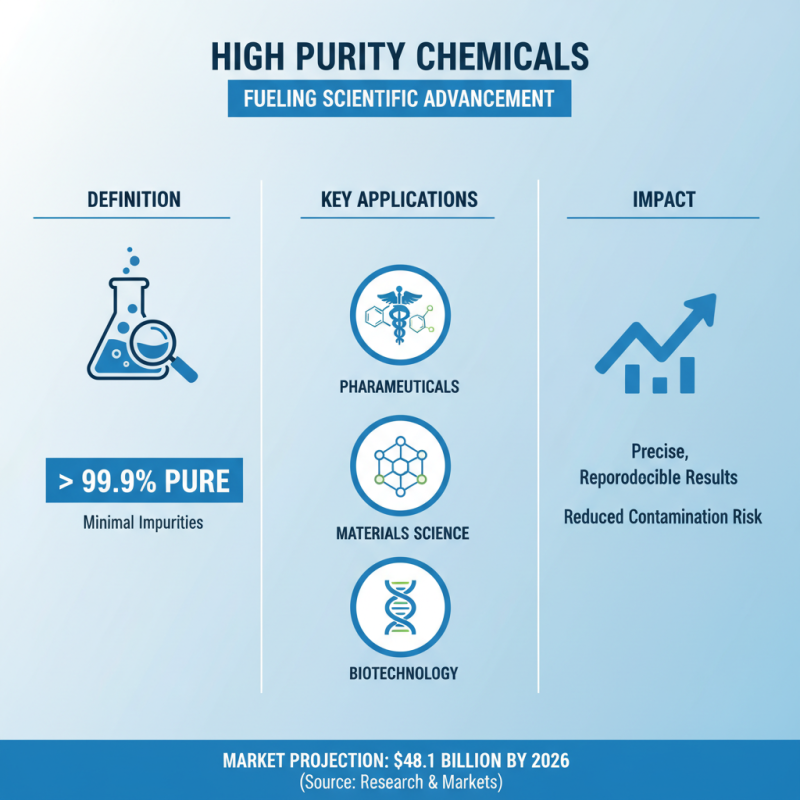
High purity chemicals play a vital role in scientific experiments, particularly in fields such as pharmaceuticals, materials science, and biotechnology. These chemicals are defined by their high degree of purity, often exceeding 99.9%, and are essential for attaining precise and reproducible results. According to a report by the Research and Markets, the high purity chemicals market is projected to reach USD 48.1 billion by 2026, highlighting the growing importance of these substances across various industries. The stringent requirements for high purity levels ensure that experimental outcomes remain consistent and valid, significantly reducing the risk of contamination that can lead to erroneous conclusions.
The significance of using high purity chemicals extends beyond accuracy; they also facilitate advancements in innovative research. For instance, in pharmaceutical development, the presence of impurities can affect drug efficacy and safety. A study published in the Journal of Pharmaceutical Sciences demonstrated that using high purity solvents reduced variability in assay results by up to 30%, underscoring their critical role in achieving reliability in research outcomes. By investing in high purity chemicals, laboratories can enhance the integrity of their studies, ultimately leading to more credible results and boosting confidence in scientific findings.
Identifying Sources and Suppliers of High Purity Chemicals
When it comes to sourcing high purity chemicals for your experiments, identifying reliable suppliers is crucial. Start by researching suppliers that specialize in high purity chemicals. Look for those that provide detailed product specifications, including purity levels, batch numbers, and certifications. Online catalogs and scientific supply platforms often feature user reviews and ratings, which can help gauge a supplier's reliability.
Tips: Always verify the supplier’s reputation in the scientific community. Check for compliance with industry standards such as ISO certifications. Engaging with forums and communities of researchers can provide valuable insights and recommendations regarding reputable suppliers.
Tips: Establish a good relationship with your supplier; this can lead to better pricing and quicker access to new products. Keep an eye out for webinars or informational sessions they may provide, as these can further enhance your understanding of the chemicals and their applications.
Evaluating the Purity Levels of Chemicals for Your Specific Needs
When evaluating the purity levels of chemicals for your specific needs, it is crucial to begin with a clear understanding of the required purity for your experiments. Different applications may demand varying levels of purity; for instance, analytical chemistry applications often require ultra-high purity chemicals with a purity level of 99.99% or higher, while general laboratory use may only need chemicals with a purity of 95% or greater. Defining these criteria allows researchers to effectively narrow down their options and avoid costly mistakes associated with using inadequate materials.
Once you have established the necessary purity levels, the next step involves assessing the certificates of analysis (CoA) and specifications provided by suppliers. A CoA not only confirms the purity of the chemical but also outlines any impurities present, offering insight into how these might affect your experiments. It is also essential to consider the method of purification, as different processes can impact the overall quality and stability of the chemicals. By carefully evaluating these aspects, researchers can ensure that they select high-purity chemicals best suited for their specific experimental conditions, thereby enhancing the reliability and accuracy of their results.
How to Choose and Use High Purity Chemicals for Your Experiments
| Chemical Name | Purity Level (%) | CAS Number | Usage/Application | Storage Conditions |
|---|---|---|---|---|
| Acetic Acid | 99.7 | 64-19-7 | Reagent in organic synthesis | Store in a cool, dry place |
| Sodium Chloride | 99.5 | 7647-14-5 | Biological and chemical applications | Keep in a tightly closed container |
| Ethanol | 99.8 | 64-17-5 | Solvent, disinfectant | Store away from heat and flames |
| Hydrochloric Acid | 37 | 7647-01-0 | pH adjustment, cleaning agent | Store in a cool, well-ventilated area |
| Potassium Nitrate | 99.0 | 7757-79-1 | Fertilizer, food preservation | Keep dry and away from moisture |
Proper Handling and Storage Practices for High Purity Chemicals
When working with high purity chemicals, proper handling and storage practices are crucial to maintain their integrity and prevent contamination. First and foremost, always wear appropriate personal protective equipment (PPE), including gloves, goggles, and lab coats, to minimize exposure to hazardous materials. It is essential to work in a clean, designated area free from potential contaminants. Use dedicated tools and equipment that have been cleaned and, if necessary, rinsed with the high purity solvent or chemical you are working with, thereby ensuring that residual impurities do not compromise your experiments.
In terms of storage, high purity chemicals should be kept in well-sealed containers that are clearly labeled with relevant information, including the chemical name, concentration, and safety data. Store them in a controlled environment, ideally away from light, moisture, and temperature variations, which can affect their stability. For certain chemicals, refrigeration might be appropriate, while others may require room temperature storage. Regularly check stock for any signs of degradation or contamination, and promptly dispose of any outdated or compromised chemicals following your organization’s safety procedures. Additionally, maintaining an organized inventory and using a first-in, first-out approach can further enhance the longevity and reliability of high purity chemicals in your lab.
Best Practices for Using High Purity Chemicals in Experimental Procedures
When using high purity chemicals in experimental procedures, it is crucial to follow best practices to ensure accuracy and reliability of results. First and foremost, proper storage is essential. High purity chemicals should be stored in their original containers with securely sealed lids to prevent contamination. It is important to keep them in a cool, dark place, away from direct sunlight or extreme temperatures, as these conditions can degrade their effectiveness. Additionally, always check the expiration dates and inspect the chemicals for any signs of degradation before use.
Another vital aspect of working with high purity chemicals is maintaining a clean working environment. This includes using dedicated tools and equipment that have been thoroughly cleaned to avoid cross-contamination. Implementing a systematic approach to measurement, such as using calibrated scales and pipettes, will help ensure precision. It’s also advisable to prepare solutions under a fume hood or in a controlled environment to minimize exposure to contaminants. When disposing of any waste, ensure that it is done following appropriate safety protocols to maintain a safe lab environment.
Lastly, documenting all procedures and observations is key when working with high purity chemicals. This includes noting the batch numbers, concentrations, and any deviations from standard protocols. Accurate documentation not only helps in replicating experiments but also in troubleshooting any issues that may arise during the experimental process. By adhering to these best practices, researchers can maximize the integrity of their work and foster reliable outcomes.
Related Posts
-
How to Choose the Best Chemical Products for Your Business in 2025
-

10 Essential Tips for Safely Handling Chemistry Chemicals in the Lab
-
Top 10 Essential Chemicals You Need to Know for Everyday Use
-

Top 10 Innovations in Chemical Processing Driving Industry Efficiency in 2023
-

2025 Top 5 Innovative Chemical Solutions for Sustainable Industry Growth
-

2025 How to Choose the Right Chemical Products for Your Business


