
Careers
How to Safely Use Nitric Acid in Your Laboratory?
Nitric acid is a crucial chemical used in many laboratories. It serves various purposes, including making fertilizers and explosives. According to a 2022 report by the Global Chemical Safety Board, nitric acid-related incidents have risen by 15% in recent years, highlighting the need for safety measures.
Proper handling of nitric acid is vital. This strong acid can cause severe burns and respiratory issues. Reports indicate that almost 30% of laboratory accidents involve chemical exposure. A safe laboratory environment requires understanding the risks associated with nitric acid. Using appropriate personal protective equipment, such as gloves and goggles, is essential.
Despite safety training, human error can occur. Even seasoned professionals may overlook safety protocols. Regular drills and equipment checks help mitigate these risks. Each laboratory should establish clear guidelines for handling nitric acid. Continuous education on the acid's dangers plays a critical role in promoting a culture of safety.

Understanding Nitric Acid and Its Properties in Laboratory Use
Nitric acid is a strong corrosive acid used widely in laboratories. It is colorless with a pungent odor. Understanding its properties is crucial for safe handling. Its molecular formula is HNO₃, and it has a boiling point of 83°C. In concentrated form, it can cause severe burns on contact with skin. Proper lab practices should always be in place when working with this chemical.
According to the National Institute for Occupational Safety and Health (NIOSH), inhaling nitric acid vapors can irritate the respiratory tract. Even short exposure can lead to coughing or difficulty breathing. The acid is also a strong oxidizer, so it reacts dangerously with many substances. In practice, this means labs must store nitric acid properly, away from organic materials. Using proper personal protective equipment is vital. Gloves and goggles are a must.
Contamination is a real issue. Spills should be contained quickly; however, cleanup procedures can outpace understanding. Often, labs underestimate the initial risk involved with nitric acid. Training sessions can be hit or miss. Many researchers find themselves unprepared for emergency situations. Regular reviews and drills may enhance readiness. Testing awareness fosters a safer environment. Every lab should prioritize knowledge of nitric acid properties in their safety protocols.
Usage of Nitric Acid in Laboratory Settings
This chart illustrates the various applications of nitric acid in laboratory settings, highlighting the safety levels associated with each application.
Essential Safety Equipment for Handling Nitric Acid
When handling nitric acid, safety equipment is crucial. Protective gear should be your priority. Wear safety goggles to shield your eyes from splashes. A lab coat and gloves are essential, too. Choose gloves made of nitrile or another chemical-resistant material.
Tips: Double-check your equipment. Ensure it’s free from any rips or tears.
A fume hood is necessary when working with nitric acid. It helps avoid inhalation of harmful vapors. If a fume hood is unavailable, use a well-ventilated area. Good ventilation can greatly reduce exposure risks.
Tips: Keep emergency equipment nearby. Eye-wash stations and safety showers should be accessible. Regularly inspect them to ensure functionality.
Chemicals can be unpredictable, and accidents may happen. Always have a spill kit on hand. Practice proper disposal methods for any waste materials. This will keep your lab safe and clean.
Proper Storage and Labeling Practices for Nitric Acid
Proper storage and labeling of nitric acid are crucial in laboratory settings. According to the Occupational Safety and Health Administration (OSHA), around 30% of laboratory accidents involve chemical mismanagement. To lower this risk, it is vital to use clearly labeled containers. Labels should include the substance name, concentration, and hazards. Using color-coded labels can help in quickly identifying nitric acid.
Storage conditions matter as well. Nitric acid should be kept in a cool, ventilated area away from incompatible substances, like organic materials. Ideally, it should be stored in a dedicated chemical storage cabinet. However, many labs overlook this crucial detail. Some leave chemicals in less secure places, leading to potential spills or reactions. Regular audits can help ensure that nitric acid is stored properly.
Proper practices also extend to training personnel. According to a study published in the Journal of Chemical Health and Safety, lack of training is a common cause of laboratory incidents. Staff should understand the risks associated with nitric acid and how to respond in emergencies. Incorrect labeling or storage can lead to dangerous situations. Reflecting on these practices can highlight areas needing improvement.
How to Safely Use Nitric Acid in Your Laboratory? - Proper Storage and Labeling Practices for Nitric Acid
| Storage Condition | Recommended Container | Labeling Requirements | Safety Precautions |
|---|---|---|---|
| Cool, dry place away from sunlight | Glass or high-density polyethylene container | Include hazard symbols, concentration, and handling instructions | Wear gloves and goggles; use fume hood |
| Locked cabinet for chemicals | Corrosion-resistant container | State "Corrosive" and "Toxic" clearly | Avoid contact with skin or eyes |
| Away from incompatible substances | Sealable, non-reactive caps | Date of purchase and expiration date | Store in ventilation for gas release |
Step-by-Step Procedures for Safe Nitric Acid Usage
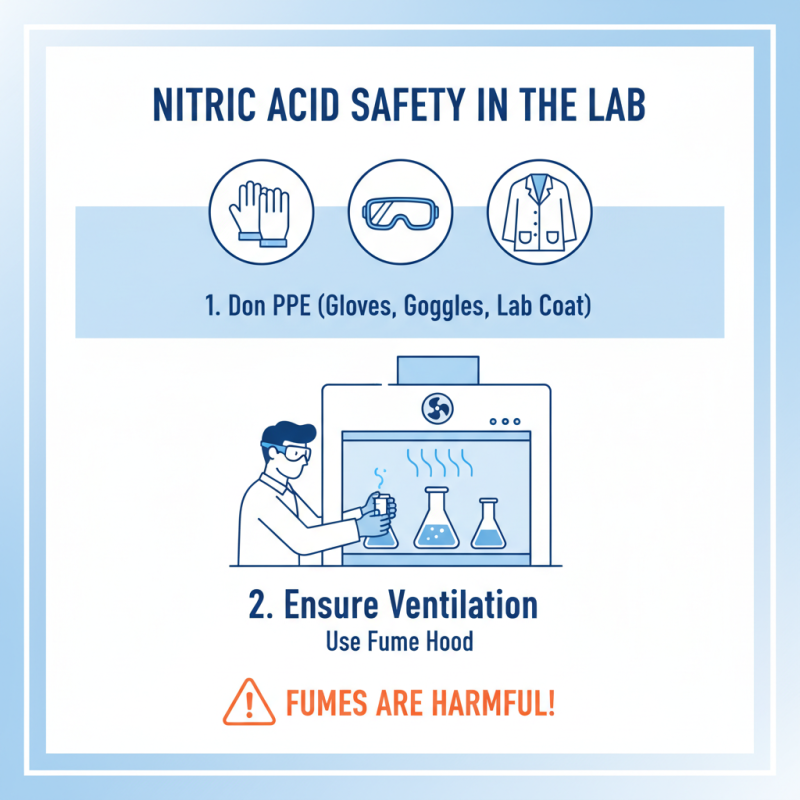
Using nitric acid safely in the laboratory requires attention to detail. Begin by donning appropriate personal protective equipment. This includes gloves, goggles, and a lab coat. These items protect against accidental spills. Ensure your workspace is well-ventilated. Use a fume hood if possible. Nitric acid fumes can be harmful, so don’t take this lightly.
When handling nitric acid, always pour it slowly. This will minimize the risk of splashing. Use a designated container that is resistant to corrosion. Avoid mixing it with other chemicals unless you fully understand the reactions. Mistakes can lead to dangerous outcomes, so be mindful. Label all containers clearly to prevent confusion.
After using nitric acid, clean up spills immediately. Use neutralizing agents to safely manage any residues. Dispose of waste according to your facility's protocols. Reflect on the process to improve your handling of hazardous materials. Accidents can happen, and it’s important to learn from them. Laboratory safety is a continuous journey, not a destination.
Emergency Response Protocols for Nitric Acid Incidents

In the event of a nitric acid spill, immediate action is crucial. Begin by evacuating the area to ensure everyone's safety. Notify the appropriate personnel about the incident. Protect yourself with personal protective equipment such as goggles, gloves, and a lab coat. This will reduce the risk of injury while handling the situation.
Contain the spill using absorbent materials designed for acids. Avoid using products that can react negatively with nitric acid. If the acid splashes onto skin or clothing, rinse the affected area with plenty of water for at least 15 minutes. It's a simple step, but many overlook it in the chaos of a spill. Be mindful of fumes as they can be harmful in confined spaces. Ventilate the area effectively if safe to do so.
After the initial response, document the incident thoroughly. Reflecting on what went wrong can prevent future occurrences. Review safety protocols with your team regularly. Training sessions help maintain awareness. Often, people forget the basics in routine tasks. Regular reminders can reinforce safe practices in the lab.
Related Posts
-

10 Essential Tips for Safely Handling Pure Nitric Acid in Laboratories
-
Top 10 Tips for Choosing the Right Industrial Chemical Packaging Solutions
-

10 Essential Tips for Safely Handling Chemistry Chemicals in the Lab
-

How to Choose and Use High Purity Chemicals for Your Experiments
-

2025 Top 5 Innovative Chemical Solutions for Sustainable Industry Growth
-

How to Optimize Manufacturing Chemical Processes for Better Efficiency


